Solid Phase Peptide synthesis Mechanism
Peptide synthesis is the production of peptide. Over the year different processes and methods were discovered and invented to produce a large number of peptides to meet the need of the protein in different areas of medical sciences. The organic chemistry has helped a great deal in peptide synthesis mechanism by which peptides are produced. Peptide synthesis is robust and foolproof. However, there are certain things which can really disturb the reproducibility of these protocols. Probably the chief amongst all disturbing elements is the quality of DMF. It is incredibly important to use ‘quality’ DMF during the solid phase peptide synthesis to achieve better yield. This means either getting it off the solvent system or opening a new bottle. There are few solid phase peptide synthesis mechanisms that fall under the solid phase peptide synthesis.
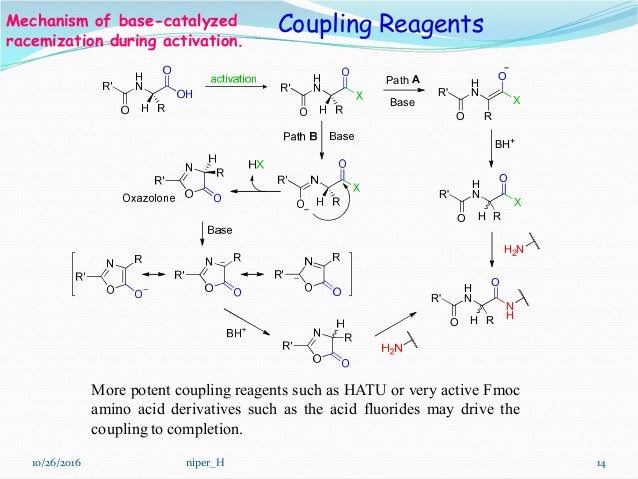
The first stage in solid-phase peptide synthesis is the choice; choosing what functional group you want your C -terminus to be:
If you want your C -terminus to be a carboxylic acid use 2-chlorotrityl resin.
If you want your C -terminus to be an amide use Rink amide resin.
If you are making a macrocyclic peptide use 2-chlorotrityl resin.
Once your choice of resin is made you will need to load your first amino acid onto the resin.
1- The process constitutes weighing up of the appropriate amount of resin. Generally, 300 mg for a 0.1 mmol
scale synthesis is used. Unload the resin into a Poly-Prep chromatography column (BioRad).
2- Let resin swell for at least 30 min (longer is okay) at room temperature in CH2Cl2.
3- Weigh out an appropriate amount of the first amino acid and dissolve it in 8 mL CH2Cl2 w/ 0.3 ml
2,4,6-collidine. When making a macrocyclic peptide our first amino acid is almost always Boc-Orn(Fmoc)-
OH. Use ca. 100 mg of Boc-Orn(Fmoc)- OH.
4- Using a flow of nitrogen gas, push out all CH2Cl2 from the column that contains the swelled resin and adds the Amino acid/DCM/Collidine solution.
5- Rock for at least 8 hours (no longer than 24 hours).
6. Move on to capping 2-chlorotrityl Resin.
Capping 2-Cholotrityl Resin
The reason behind this step is to covalently link a small nucleophile (methanol) to the unreacted carbocations on the 2-chlorotrityl chloride resin.
Prep time: 10 mins; Reaction time: 1 hour 1.
1- Clean the loaded resins 3X with CH2Cl2.
2-After cleaning makes the capping solution using CH2Cl2: MeOH: DIPEA (17:2:1). Make this fresh each time by adding 1 ml MeOH and 0.5 ml diisopropylethylamine (DIPEA, or DIEA) to 9 ml of CH2Cl2.
3- Load off the capping solution on to the loaded resin and rock for 1 hour at room temperature. Do not extend the reaction time more than suggested, as an exchange of the loaded amino acid with MeOH is a possibility.
4- After 1 hour, drive out the capping solution with nitrogen and wash the resin 2X with CH2Cl2 and 1X with DMF. It is for you to analyze as to how efficient your resin was loaded. Usually, this step is overlooked, though, as loading 2-chlorotrityl resin is VERY reproducible if you do not stray from the protocol detailed above.
5- The loaded resin is all set to go through repeated Fmoc-deprotection and amino acid couplings to produce the rest of your peptide. The process of deprotection and coupling can be easily done manually (hand coupling) or on an automated synthesizer.
For peptide Synthesis visit on - Custom Peptide Synthesis

The first stage in solid-phase peptide synthesis is the choice; choosing what functional group you want your C -terminus to be:
If you want your C -terminus to be a carboxylic acid use 2-chlorotrityl resin.
If you want your C -terminus to be an amide use Rink amide resin.
If you are making a macrocyclic peptide use 2-chlorotrityl resin.
Once your choice of resin is made you will need to load your first amino acid onto the resin.
1- The process constitutes weighing up of the appropriate amount of resin. Generally, 300 mg for a 0.1 mmol
scale synthesis is used. Unload the resin into a Poly-Prep chromatography column (BioRad).
2- Let resin swell for at least 30 min (longer is okay) at room temperature in CH2Cl2.
3- Weigh out an appropriate amount of the first amino acid and dissolve it in 8 mL CH2Cl2 w/ 0.3 ml
2,4,6-collidine. When making a macrocyclic peptide our first amino acid is almost always Boc-Orn(Fmoc)-
OH. Use ca. 100 mg of Boc-Orn(Fmoc)- OH.
4- Using a flow of nitrogen gas, push out all CH2Cl2 from the column that contains the swelled resin and adds the Amino acid/DCM/Collidine solution.
5- Rock for at least 8 hours (no longer than 24 hours).
6. Move on to capping 2-chlorotrityl Resin.
Capping 2-Cholotrityl Resin
The reason behind this step is to covalently link a small nucleophile (methanol) to the unreacted carbocations on the 2-chlorotrityl chloride resin.
Prep time: 10 mins; Reaction time: 1 hour 1.
1- Clean the loaded resins 3X with CH2Cl2.
2-After cleaning makes the capping solution using CH2Cl2: MeOH: DIPEA (17:2:1). Make this fresh each time by adding 1 ml MeOH and 0.5 ml diisopropylethylamine (DIPEA, or DIEA) to 9 ml of CH2Cl2.
3- Load off the capping solution on to the loaded resin and rock for 1 hour at room temperature. Do not extend the reaction time more than suggested, as an exchange of the loaded amino acid with MeOH is a possibility.
4- After 1 hour, drive out the capping solution with nitrogen and wash the resin 2X with CH2Cl2 and 1X with DMF. It is for you to analyze as to how efficient your resin was loaded. Usually, this step is overlooked, though, as loading 2-chlorotrityl resin is VERY reproducible if you do not stray from the protocol detailed above.
5- The loaded resin is all set to go through repeated Fmoc-deprotection and amino acid couplings to produce the rest of your peptide. The process of deprotection and coupling can be easily done manually (hand coupling) or on an automated synthesizer.
For peptide Synthesis visit on - Custom Peptide Synthesis


Oligopeptides, also known as small peptides, are bioactive peptides composed of 2-10 amino acid residues linked by peptide bonds. Numerous studies have shown that proteins are eventually absorbed in the form of oligopeptides and amino acids, Oligopeptide Synthesis
ReplyDeletesitessolutionreview http://sitessolutionreview.blogspot.com/
ReplyDeletebeautyandfashionideas http://beautyandfashionideas.blogspot.com/
fitnessandweightlossguide http://fitnessandweightlossguide.blogspot.com/
healthandbeautypros http://healthandbeautypros.blogspot.com/
mensfashionideas http://mensfashionideas.blogspot.com/
onlineshoppingtipsandtricks http://onlineshoppingtipsandtricks.blogspot.com/